|
Endeavor Stent: 5-Year
Data Show Safety and Efficacy Advantage
|
 |

Medtronic's
Endeavor® Stent |
|
March 14, 2010 --
The presentation at today's American College of Cardiology meeting
of the 5-Year clinical results from ENDEAVOR III, along with
the results of Medtronic's
(NYSE: MDT) pooled Endeavor Trials Program, offers what
may be a new paradigm for judging clinical trial results. Especially
when taken together with recent findings from such studies as
FAME and ODESSA, angiographic late lumen loss, one of the standards
by which the performance of drug-eluting stents initially has
been judged, may not be as significant a factor as once thought. |
Late Lumen Loss
Late lumen loss (LLL) is defined
as the difference in millimeters in the diameter of a stented arterial
segment
immediately
post-procedure
with the diameter of the same segment six to nine months later. The
amount of tissue regrowth has been seen as a surrogate for the effectiveness
of the
drug-eluting stent. Logically, the greater the tissue regrowth, the
higher chance for restenosis, return of symptoms or, in the worst
case scenario, heart attack or death.
Early Results for Endeavor
When the initial clinical
trials of the Endeavor zotarolimus-eluting stent were first presented
in
2005,
there was
concern in the interventional
community over the greater late loss, compared with
other drug-eluting stents, even prompting Dr. Marie-Claude Morice
to dub it "...the stent of les gens paresseux [lazy
people]" because it was easy to deliver and the procedure could
be faster but, she stated, "...it is not in the running to be
compared favorably with the two others [Taxus and Cypher]." [As
quoted in theheart.org,
March 7, 2005] The ENDEAVOR III eight-month
data, in fact, failed to achieve its primary
endpoint
of non-inferiority with the Cypher regarding late loss: the late
loss was four times greater with the Endeavor.
| Five-Year
Results Show Shift |
|
|
| Now fast forward to five years
later and some interesting changes have taken place. The question
has always been, "Does increased late loss predict worse clinical
outcomes." As seen by this chart, part of today's presentation
of the ENDEAVOR III 5-Year Clinical Results, the answer would
be probably not. Even though the Cypher had much less
late loss at eight months, its rate of Major Adverse Clinical
Events
(death, heart attack, and the need to reopen the stent) exceeded
Endeavor's somewhere close to the two year mark and, more
importantly, continued to grow up through five years with no
sign of leveling off -- adverse outcomes are 60% greater. |
|
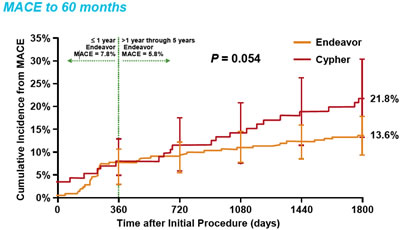
ENDEAVOR
III 5-Year Clinical Results |
This phenomenon, known as late catch-up, was most recent seen in
SIRTAX-LATE, a study presented at TCT 2009. Again the Cypher had
shown to be superior to the Taxus stent at one year, but at five,
the two stents were virtually equivalent in results. Interestingly,
the Cypher crossover in SIRTAX-LATE also occurred right around the
two year mark.

David E.
Kandzari, MD |
|
Dr. David Kandazari of the
Scripps Clinic, lead investigator for the ENDEAVOR III
trial, told Angioplasty.Org that ENDEAVOR III represents a
frame-shift
in thinking about DES trials:
"Just as in clinical practice, in trials
there is life beyond ascertainment of the primary endpoint.
Events continue to accrue both with regard to safety and
failure
of efficacy in these patients. Now
that we're in an era of comparative DES trials, were seeing
emerging differences in both safety and efficacy outcomes
-- emerging differences that extend well beyond our
initial preoccupation with the angiographic measures like
late loss...that paradigm has completely shifted now
to more appropriate focus on patient-level outcomes of endpoints
like death, myocardial infarction and target lesion revascularization."
|
Safety
One of the major concerns about drug-eluting stents has been
the specter of late stent thrombosis (LST), a phenomenon first
brought to light during the 2006 European Society of Cardiology
meeting.
Signals were discerned that after one year, supposedly well after
the metal struts of stents had been covered by healthy endothelial
cells, blood suddenly would clot inside the stent, causing a
thrombosis which 50% of the time led to heart attack and possibly
death. At the time, only
the Cypher and Taxus stents were on the market, but the fears
over these late-occurring events drove down the use of
DES from 90% to the low 60% range in less than a year. Ever since,
the late
stent
thrombosis rate has been an integral part of clinical trials
-- and most show that it is a very infrequent event, but one
which seems to increase at a low rate annually.
| Some recent studies
have implicated the polymer on these stents as possibly causing
late malapposition of the arterial wall, perhaps as part of a
hypersensitivity reaction. However, as seen in this chart of
the 5-Year Pooled Analysis of the entire Endeavor Program (over
2,000 patients) the late stent thrombosis phenomenon is virtually
non-existent. Only 3 patients experienced LST after 1 year; only
1 of them occurred between year two and five. In this regard,
the Endeavor shows a safety profile similar to a bare metal stent
(in this case, the Driver) although the actual DES stent thrombosis
rate is half that of the BMS. |
|
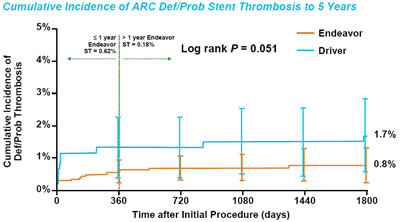
Endeavor
Program Pooled Analysis 5-Year Clinical Results |
Consistency
When following stent performance over a long-term, what is important
is that there are no "surprises", such as late catch-up
-- that after the first year, the changes are minimal. The
Pooled Analysis of the 5-Year Endeavor vs. BMS can be seen
below, and, as Dr. Kandzari
told Angioplasty.Org:
"We see a consistency
for the Endeavor stent. Although you've taken up front the higher
risk of TLR
in that first year, especially when you have a very high rate
of angiographic
follow-up...on the other
hand, beyond that surveillance angiography, the rates
of TLR seem to
be more level or plateaued, or more durable. And this
is the consistency we've seen now across all of the key
registration trials for Endeavor, over half of which
have now been followed for five years."
|
|
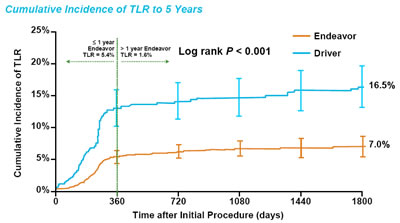
Endeavor
Program Pooled Analysis 5-Year Clinical Results |
The Endeavor program is the only "second
generation" stent to have data out to five-years at this point. The
Xience/Promus stent has reported three-year results in
the SPIRIT III (1,002 patients) presented at last year's TCT. The
significance of this long term Endeavor data is to emphasize the
concept that results at 8 months and one year are certainly important
and
interesting
-- but it is entirely possible that late term data may considerably
mediate initial impressions.
Reported by Burt Cohen, March 14, 2010
|





