GE Healthcare and Volcano Corporation Collaborate to Develop a State-of-the-Art Cath Lab System with Fully Integrated IVUS Capabilities
Collaboration Agreement Brings Together Real-Time, Leading-Edge Imaging Technologies
Waukesha, WI and Rancho Cordova, CA -- October 17th, 2005 - GE Healthcare
and Volcano Corporation today announced a significant development and collaboration
agreement that is intended to bring an industry-first imaging capability to cardiac
catheterization labs around the world. Under terms of the agreement, GE and Volcano
will collaborate to develop a state-of-the-art digital cardiovascular imaging
system with fully integrated intravascular ultrasound imaging (IVUS) capabilities.
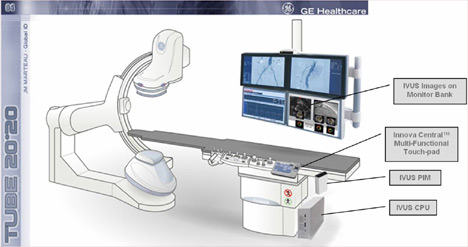
Figure 1: Schematic of IVUS components and images integrated into GE Innova Cath Lab
GE Healthcare provides transformational medical technologies that are shaping a new age of patient care. GE Healthcare's expertise in medical imaging and information technologies, medical diagnostics, patient monitoring and life support systems, disease research, drug discovery, and biopharmaceutical manufacturing technologies is helping physicians detect disease earlier and to tailor personalized treatments for patients. GE Healthcare offers a broad range of products and services that are improving productivity in healthcare and enhancing patient care by enabling healthcare providers to better diagnose and treat cancer, heart disease, neurological diseases, and other conditions.
GE Healthcare is a $15 billion unit of General Electric Company (NYSE: GE) headquartered in the United Kingdom. Worldwide, GE Healthcare employs more than 43,000 people committed to serving healthcare professionals and their patients in more than 100 countries. For more information about GE Healthcare, visit our website at www.gehealthcare.com
About Volcano Corporation
Volcano Corporation (formerly Volcano Therapeutics, Inc.) is a
privately held medical device company founded in 2001. Investors
and shareholders
include Domain Associates, Johnson and Johnson Development Corporation,
Medtronic, Ferrer, Freeman & Co. and OrbiMed Advisors.
With over 475 worldwide employees, Volcano is dedicated to providing technologies leading to optimal management of coronary artery and peripheral vascular disease. Volcano products include Intravascular Ultrasound (IVUS) systems and catheters, as well as physiology guide wires. With global distribution, Volcano is a leading provider of innovative therapy-enabling and therapy-guiding technologies to the interventional cardiology and peripheral vascular fields. For more information, please visit www.volcanocorp.com.
The development, release and timing of any features or functionality described for our products is subject to many factors both within and outside of our control and we retain sole discretion as to the specific nature and timing of market release of such features or functionality. Volcano makes no commitment, promise or legal obligation to deliver any future product or service that may be mentioned in this press release or in any other general communication.
Source: GE Healthcare and Volcano Corporation |